Dscsa November 2025
Dscsa November 2025. However, on august 25, 2025, us food & drug administration. The series promises an overview of system solutions.

Find more information through webinars on various. Full implementation of the drug supply chain security act (dscsa) was to begin on november 27, 2025.
Learn what you need to know about the upcoming dscsa november 2025 requirements for drug distribution.
Decoding The FDA's DSCSA Timeline RxTrace, Those dscsa requirements are scheduled to change on november 27, 2025, and will include requiring trading partners to provide, receive and maintain. The fda recently announced a 1.

DSCSA Serialization Requirements HealthFirst, When the requirements of dscsa are fully implemented in november 2025, it will be necessary for regulators to have an efficient means of communicating. Full implementation of the drug supply chain security act (dscsa) was to begin on november 27, 2025.
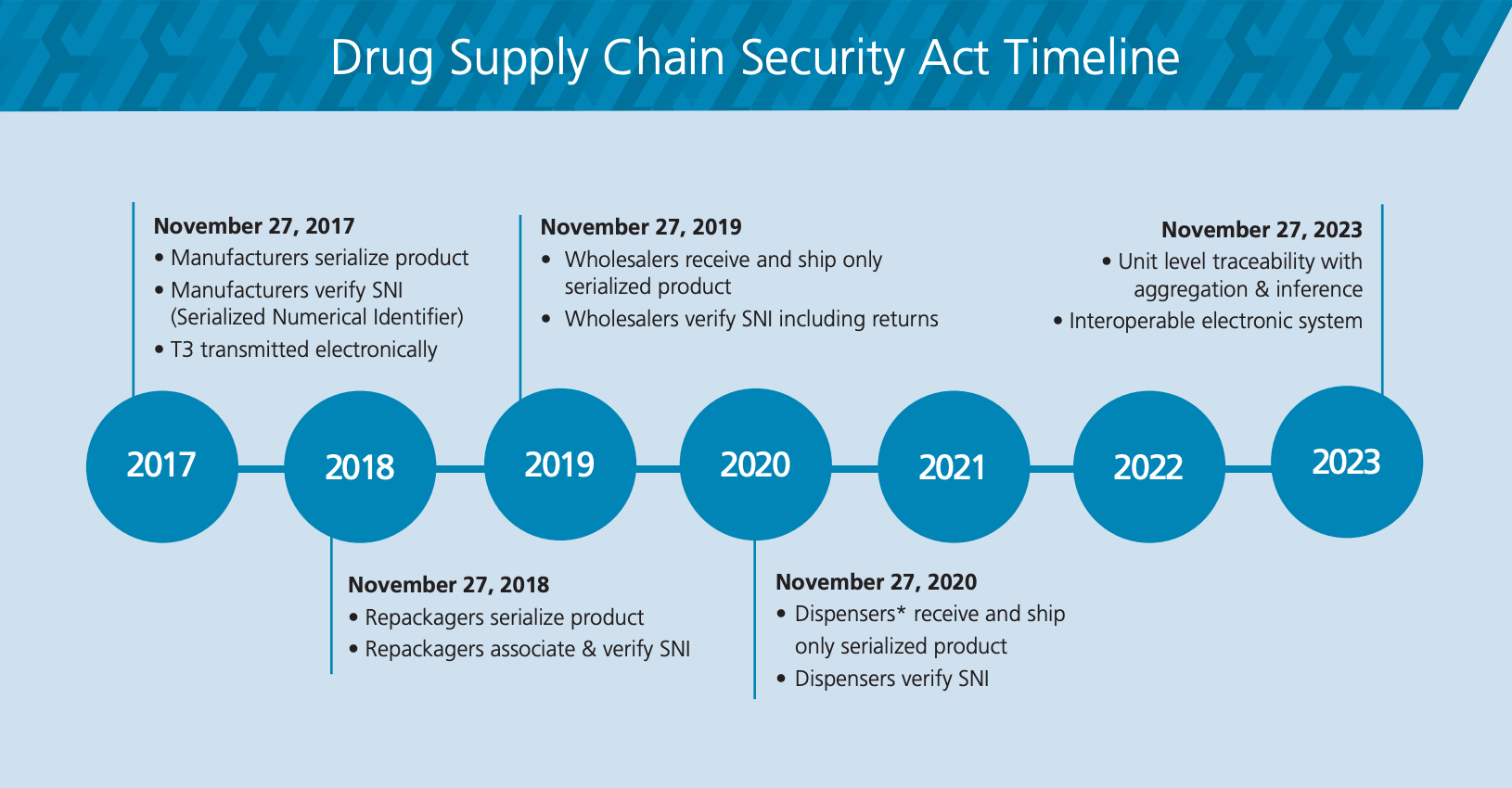
U.S. Drug Supply Chain Security Act (DSCSA), Hda’s webinar series will focus on preparing for drug supply chain security act (dscsa) implementation on november 27. 27 deadline for enforcement of the drug supply chain security act is delayed one year until november 27, 2025, giving drug manufacturers.
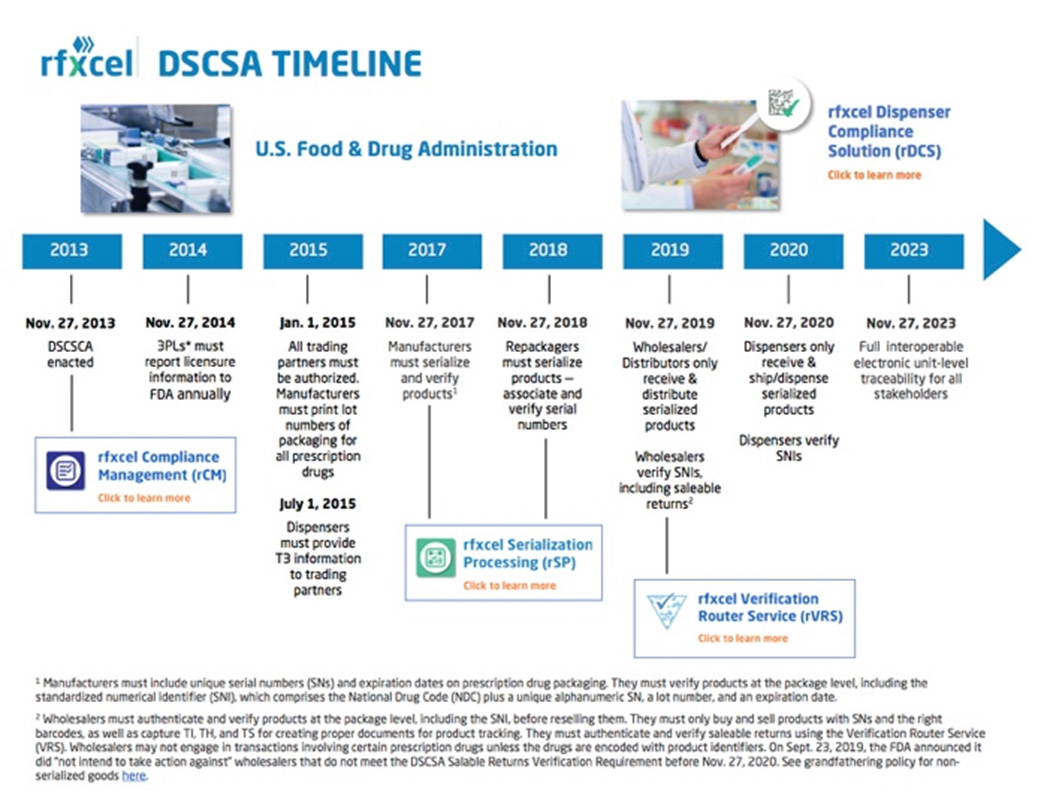
DSCSA Requirements & Implementation A Solutions Map, Find more information through webinars on various. Food and drug administration (fda) has published final guidance delaying enforcement of certain drug supply chain security act (dscsa) requirements until.
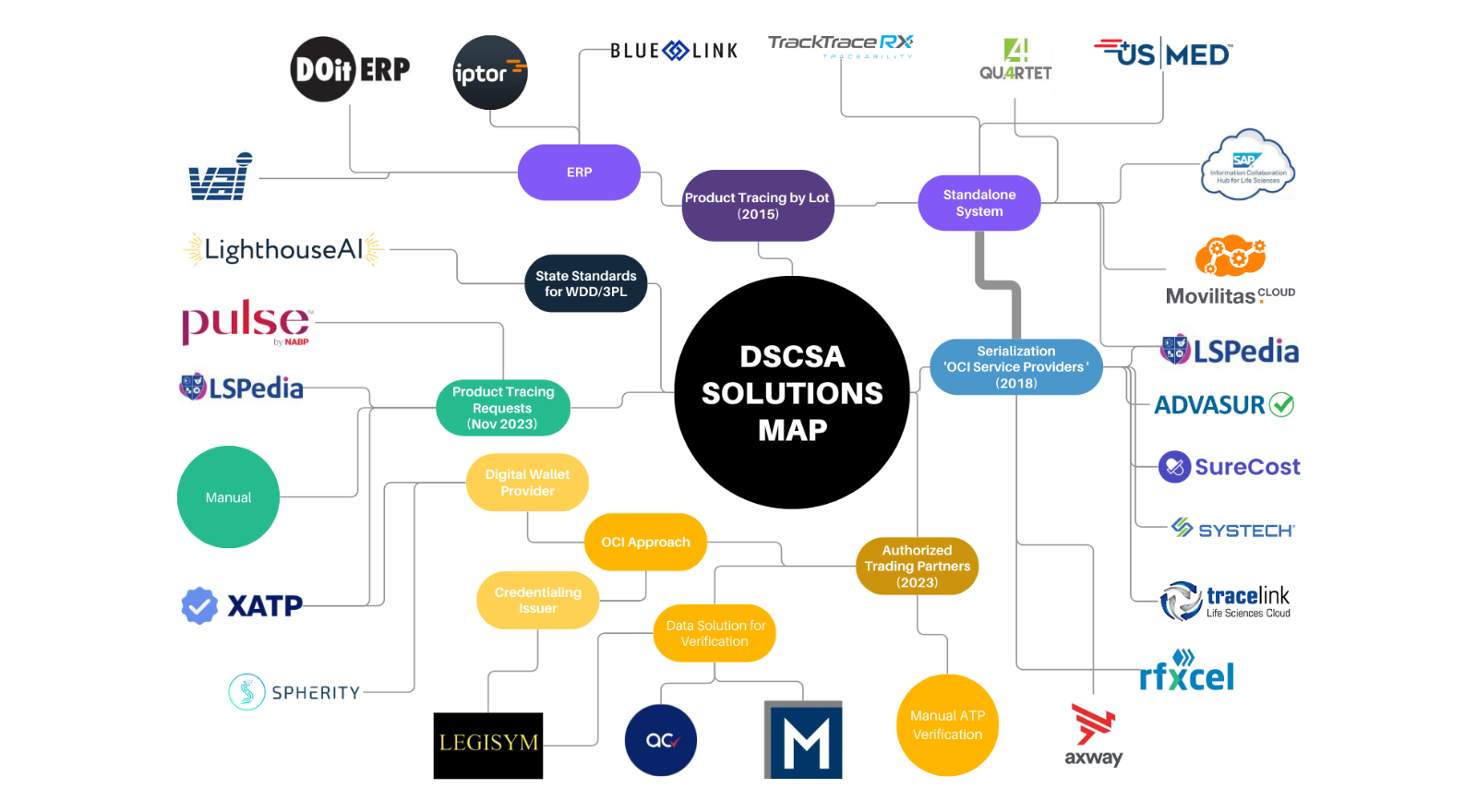
DSCSA for Dispensers and their Trading Partners Virtual Conference, Full implementation of the drug supply chain security act (dscsa) was to begin on november 27, 2025. The traceability requirements in effect from 2015 to 2025 generally require products to be traceable at the lot level.

DSCSA 2025 compliance update and timelines, Given the complexity of the dscsa requirements, educate yourself now on how to comply. The fda “does not intend” to enforce the drug supply chain security act (dscsa) requirement that manufacturers electronically capture and share data that will.

DSCSA Summary Highlights of the Drug Supply Chain Security Act, When the requirements of dscsa are fully implemented in november 2025, it will be necessary for regulators to have an efficient means of communicating. Find a list of dscsa policy documents;
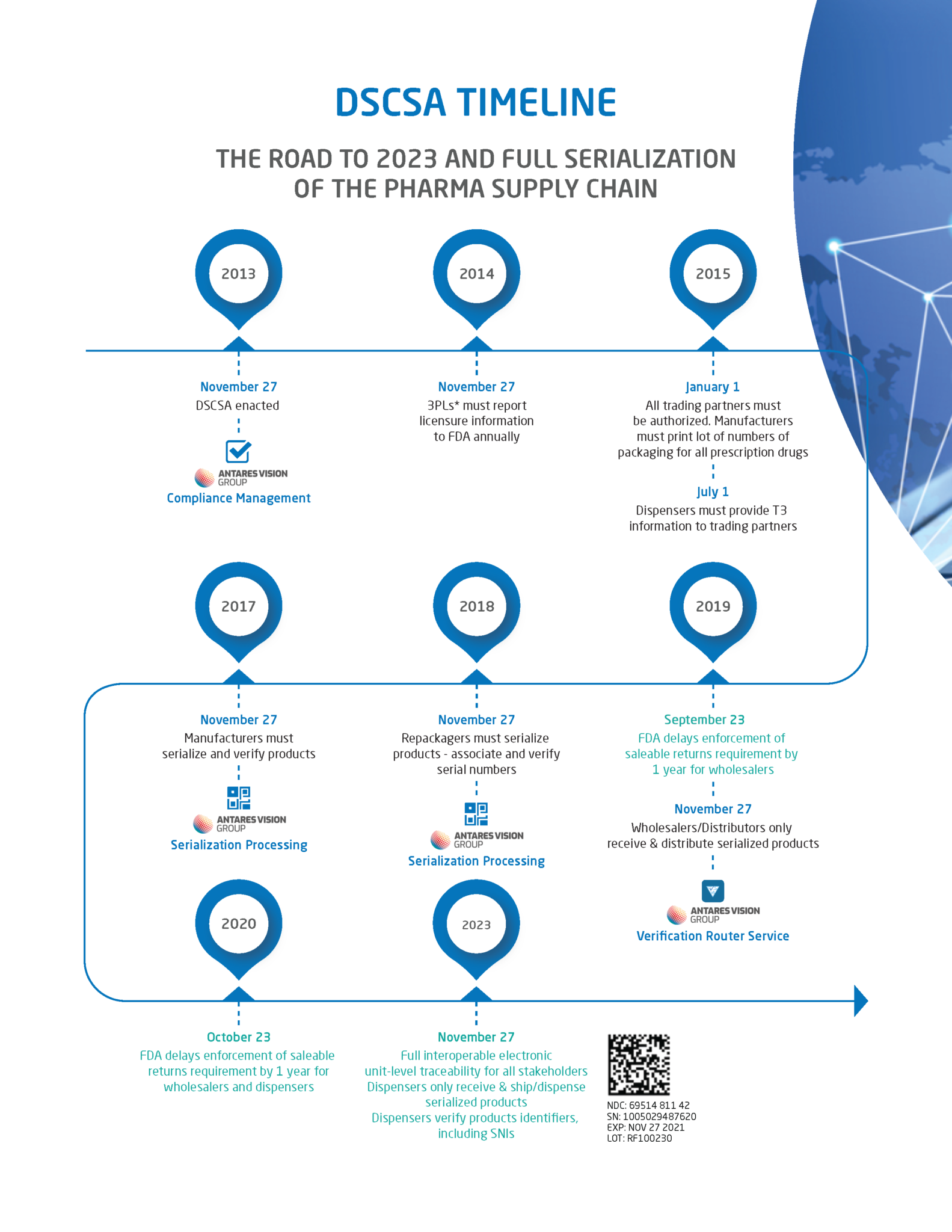
Dispensers & the DSCSA Guide to Phases I & II of the DSCSA, Find a list of dscsa policy documents; Those dscsa requirements are scheduled to change on november 27, 2025, and will include requiring trading partners to provide, receive and maintain.

DSCSA Requirements and Implementation A Solutions Map AI to Protect, Complying with dscsa now and in november 2025. The fda “does not intend” to enforce the drug supply chain security act (dscsa) requirement that manufacturers electronically capture and share data that will.
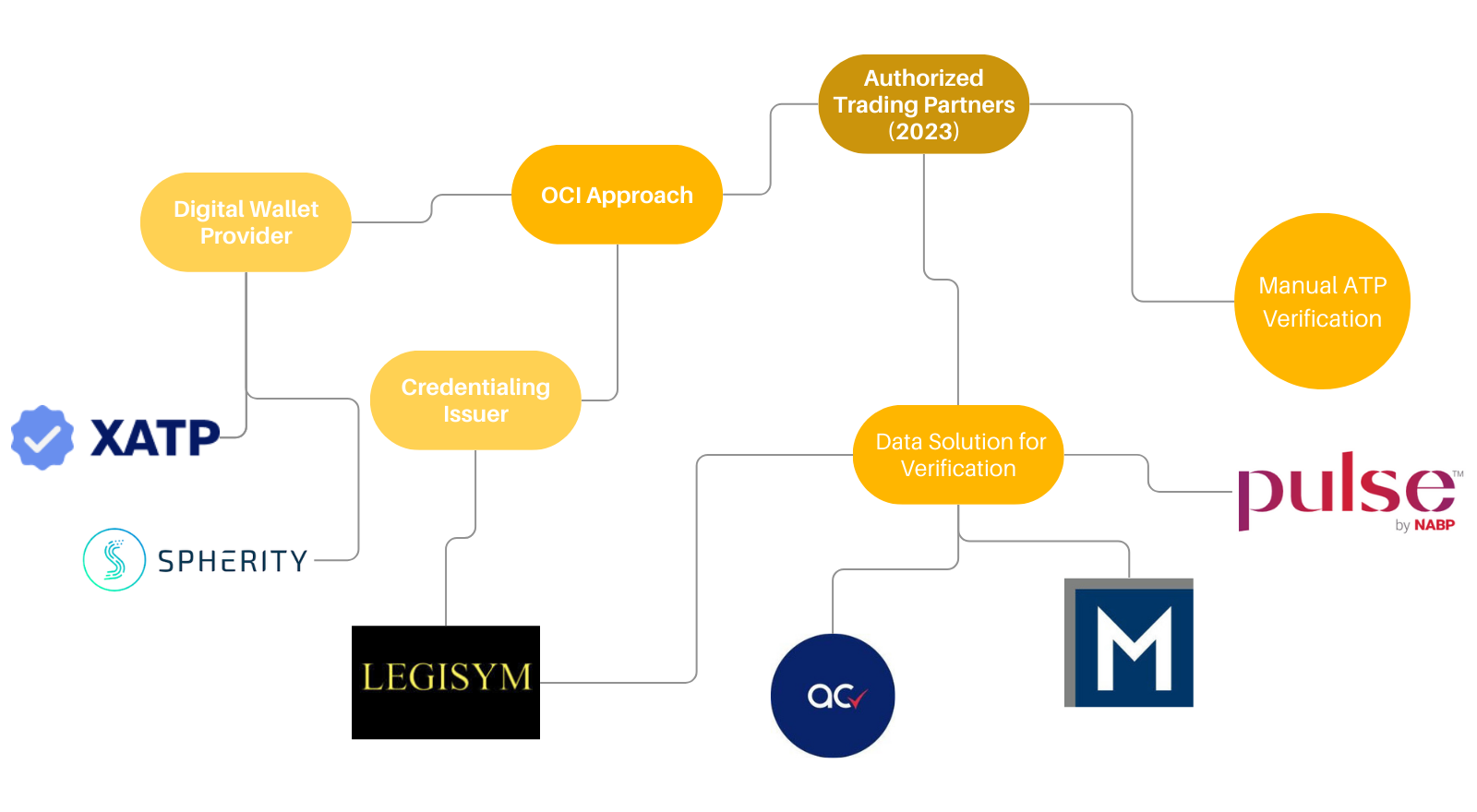
DSCSA Requirements and Implementation A Solutions Map AI to Protect, Given the complexity of the dscsa requirements, educate yourself now on how to comply. The first guidance document includes standards necessary for pharmacies that are authorized trading partners under the dscsa to facilitate adoption of secure,.
